Cancer continues to affect millions of people worldwide, but clinical trials for cancer offer hope by exploring new treatments and therapies that can potentially save lives. For individuals diagnosed with cancer, participating in a clinical trial may open doors to cutting-edge treatments and play a vital role in the advancement of medical research. In this article, we’ll explore what cancer clinical trials are, the different types available, their importance in cancer treatment, and how you can access them, especially if you’re in India or are dealing with late-stage cancer.
What Are Clinical Trials for Cancer?
Clinical trials for cancer are research studies that investigate new ways to prevent, diagnose, treat, or manage cancer. These trials are essential for evaluating new treatments, understanding side effects, and determining the best approaches to cancer care. They are typically conducted in several phases, from early-stage research (Phase I) to large-scale studies (Phase III), each designed to answer different questions about a treatment’s safety and effectiveness.
Clinical trials often offer patients access to new therapies not yet available to the general public, providing an opportunity to benefit from cutting-edge treatments. Importantly, participation in clinical trials is voluntary, and all patients receive close monitoring throughout the trial.
Purpose of a Clinical Trial for Cancer
The primary purpose of clinical trials for cancer is to explore new ways to treat cancer, improve the effectiveness of current treatments, and reduce side effects. These trials are essential for advancing medical knowledge and improving patient outcomes. Some key purposes include:
- Testing new cancer treatments: Clinical trials test innovative drugs, therapies, and medical interventions that may offer better outcomes than current treatments.
- Exploring combination therapies: Researchers investigate the effectiveness of combining different types of treatments (e.g., chemotherapy, immunotherapy, or radiation) for enhanced results.
- Improving existing treatments: Clinical trials also focus on improving the safety, effectiveness, and quality of life associated with current cancer treatments.
- Identifying personalized treatment options: Trials may help identify personalized or precision medicine options, tailoring treatment plans to the genetic makeup of the individual’s cancer.
Clinical trials help provide answers to critical questions about cancer care, and they are a key avenue for discovering the next generation of Stage 4 cancer clinical trials.
Types of Cancer Clinical Trials
Types of cancer clinical trials for cancer come in various forms, depending on the focus of the study. Some of the common types include:
- Treatment Trials: These trials test new treatments or combinations of treatments, such as drugs, chemotherapy, radiation therapy, or surgery. The goal is to determine if a new treatment works better than current standard treatments.
- Prevention Trials: Prevention trials study ways to reduce the risk of developing cancer. This might involve lifestyle changes, dietary supplements, vaccines, or medications.
- Screening Trials: Screening trials investigate methods for detecting cancer earlier, when it’s more treatable. These trials often test new ways to improve early detection of cancer.
- Quality of Life (Supportive Care) Trials: These trials focus on improving the quality of life for individuals with cancer, exploring ways to manage symptoms or side effects of treatment.
Benefits of Clinical Trials for Cancer
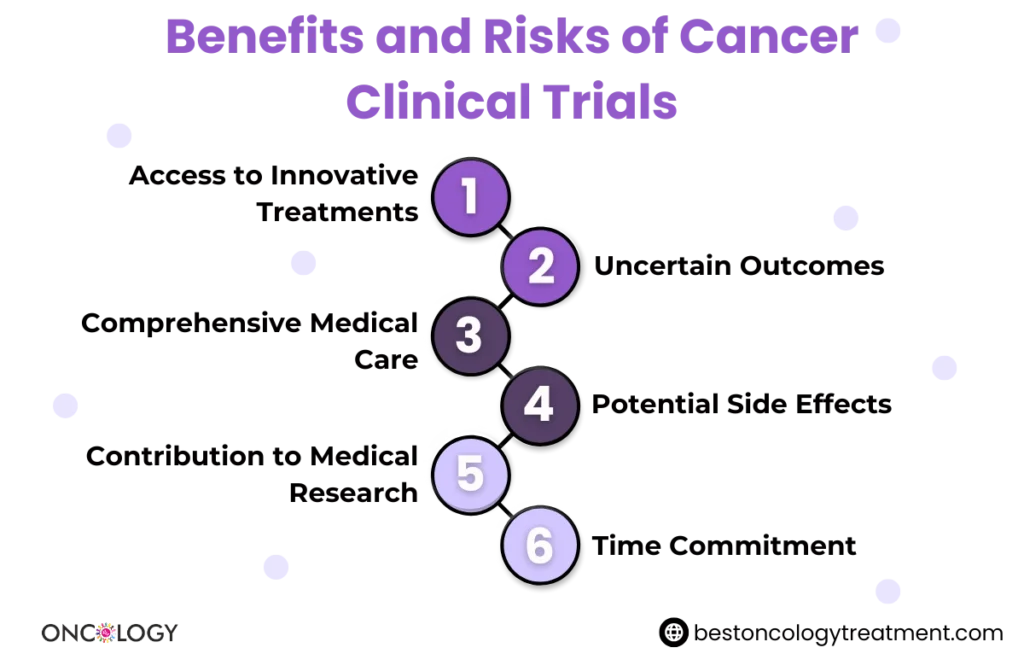
Participating in clinical trials for cancer treatment can offer a variety of benefits. These trials provide patients with access to advanced therapies and improve overall cancer care. Some of the main benefits include:
- Access to cutting-edge therapies: By enrolling in clinical trials for cancer, patients may have access to the latest treatments or experimental therapies that are not yet available in standard practice.
- Comprehensive monitoring: Participants in clinical trials receive close medical attention and regular monitoring by leading oncologists and specialists, ensuring their condition is carefully managed.
- Contributing to cancer research: Enrolling in clinical trials for cancer treatment helps advance medical science and the search for new cancer therapies, contributing to the development of new treatments for future generations.
- Improved treatment options: For patients with advanced or hard-to-treat cancer, such as Stage 4 cancer clinical trials, these trials may provide new hope and treatment options that are not yet part of conventional care.
- Enhanced quality of life: Many clinical trials focus not only on prolonging life but also on improving the quality of life by reducing symptoms and managing side effects effectively.
Participating in clinical trials for cancer in India or elsewhere can provide valuable insight into the patient’s cancer type, while also offering cutting-edge treatments for better survival and well-being.
Why Are Clinical Trials for Cancer Important?
Clinical trials are fundamental in driving progress in cancer care. Through these studies, researchers can develop new treatments that may be more effective or have fewer side effects than current options. Patients who participate in clinical trials not only gain access to these potentially life-saving treatments but also contribute to the broader understanding of cancer.
Some key benefits of cancer clinical trials include:
- Access to New Treatments: Clinical trials provide patients with access to treatments that are not yet widely available.
- Comprehensive Care: Participants receive top-notch medical care and are closely monitored by experts throughout the trial.
- Contribution to Research: By joining a clinical trial, patients help advance cancer research, contributing to future breakthroughs.
Clinical Trials for Cancer Treatment
Cancer treatment is complex, and clinical trials play a pivotal role in refining and discovering new approaches. Whether you’re newly diagnosed or in the later stages of cancer, clinical trials can offer additional options beyond standard care.
For example, individuals with Stage 4 cancer clinical trials may be able to participate in clinical trials specifically designed to target advanced disease, providing them with hope even when traditional treatments have failed. Stage 4 cancer clinical trials are often focused on innovative therapies such as immunotherapy, targeted therapies, or novel drug combinations, all aimed at improving survival and quality of life.
How to Access Clinical Trials for Cancer in India
India is emerging as a key player in clinical cancer research. The country’s medical infrastructure, along with its growing pool of research institutions, makes it easier for patients to participate in clinical trials. Clinical trials for cancer in India offer both local and international patients access to groundbreaking treatments at a relatively lower cost.
To find Clinical trials for cancer in India, consider the following steps:
- Consult with Your Oncologist: Oncologists often have access to information about ongoing trials.
- Search Online Databases: Websites like the National Cancer Institute and ClinicalTrials.gov list open trials worldwide, including those available in India.
- Reach Out to Cancer Research Institutions: Many hospitals and cancer research centers in India actively conduct clinical trials.
Are You Eligible for a Cancer Clinical Trial?
Eligibility criteria for clinical trials vary depending on the type of trial, the phase, and the specific focus of the study. Common eligibility factors include:
- Type of cancer and stage of cancer
- Previous treatments and medical history
- Overall health condition
Before enrolling in a trial, participants must undergo a detailed screening process to ensure they meet the necessary criteria. Discussing these options with your healthcare provider is crucial to determining whether a clinical trial is right for you.
The Phases of Clinical Trials
Understanding the different phases of cancer clinical trials is important for potential participants:
Phase I: Safety and Dosage
- Objective: To determine the safe dosage and potential side effects.
- Participants: Small group of 15–30 people.
Phase II: Effectiveness
- Objective: To test whether the treatment works on specific cancers.
- Participants: Larger group of around 100 or more.
Phase III: Comparison to Standard Treatments
- Objective: To compare the new treatment against the current standard.
- Participants: Thousands of patients to gather more data on safety and effectiveness.
Phase IV: Post-Market Surveillance
- Objective: To collect more information on the long-term effects and efficacy after approval.
- Participants: Patients who receive the treatment after its approval.
Key Considerations Before Joining a Clinical Trial
While clinical trials can provide promising treatments, they also come with risks. Participants should be fully informed about:
- Potential side effects
- Length of the trial
- Financial implications (some trials are fully funded, while others may involve out-of-pocket expenses)
- Expectations and commitments (such as regular visits, tests, or treatments)
Who Should Enroll in a Clinical Trial for Cancer?
Not everyone is eligible for every clinical trial for cancer. The decision to enroll depends on the type and stage of cancer, the patient’s health, and the specific eligibility criteria of each trial. Some of the individuals who may benefit from enrolling in a clinical trial for cancer treatment include:
- Patients with advanced cancer: Those with Stage 4 cancer clinical trials or other advanced cancers may be eligible for trials testing new therapies that offer better treatment options than current standard treatments.
- Those with cancer that has not responded to treatment: If a cancer patient’s condition isn’t improving with traditional therapies, they may find new options in clinical trials for cancer treatment.
- Patients seeking novel treatments: Those who are interested in exploring innovative treatments that aren’t available through conventional channels may benefit from clinical trial participation.
- Individuals with specific genetic mutations: Some trials are tailored to patients with certain genetic profiles, such as those targeting specific mutations in cancers.
- Patients who want to contribute to research: Some people choose to enroll in clinical trials to help advance the understanding of cancer and improve future cancer care for others.
Types of cancer clinical trials vary widely, and the best fit depends on the type of cancer, patient needs, and trial specifics. In clinical trials for cancer in India, there is a growing number of studies focusing on different cancer types, such as breast, lung, and colorectal cancers.
Conclusion
Joining a clinical trial for cancer is a deeply personal decision that offers numerous potential benefits. Whether you’re looking for innovative treatments or seeking to contribute to the advancement of medical research, participating in a trial could be a viable option. Discussing with your oncologist and exploring available trials can help you make an informed choice.
Check out Breast Cancer Clinical Trials.




